| Strain Name |
C57BL/6-Cd3etm2(CD3E)Bcgen/Bcgen
|
Common Name | B-hCD3E mice |
| Background | C57BL/6 | Catalog number | 110008 |
|
Related Genes |
CD3E (CD3E molecule) | ||
|
NCBI Gene ID |
12501 |
||
mRNA expression analysis
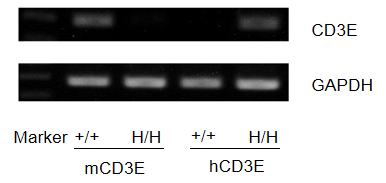
Protein expression analysis
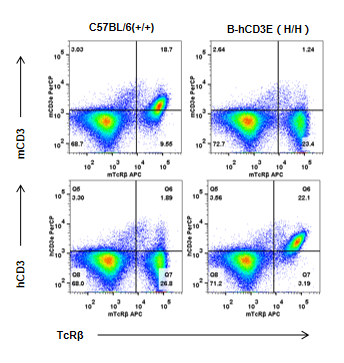
Strain specific CD3E expression analysis in homozygous B-hCD3E mice by flow cytometry. Splenocytes were collected from WT and homozygous B-hCD3E mice, and analyzed by flow cytometry with species-specific anti-CD3E antibody. Mouse CD3E was exclusively detectable in WT mice. Human CD3E were exclusively detectable in homozygous B-hCD3E but not WT mice.
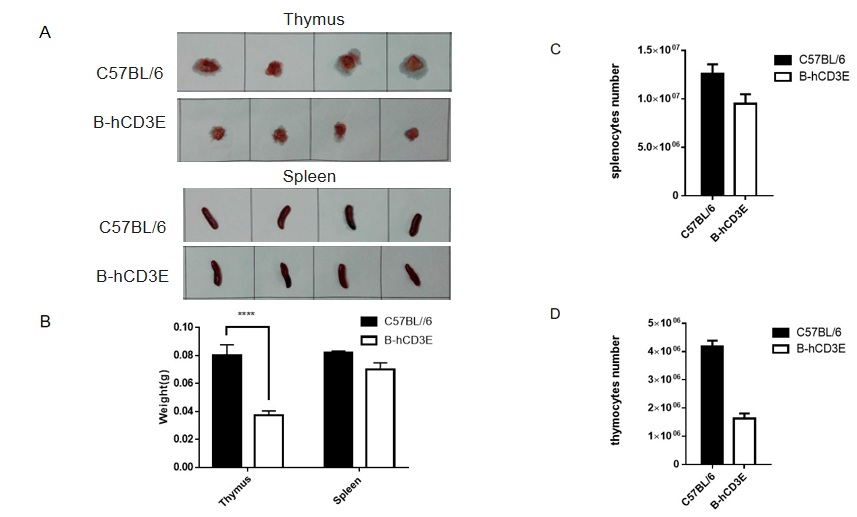
Weight of thymus and spleens and the total cell number of thymocytes and splenocytes in B-hCD3E mice
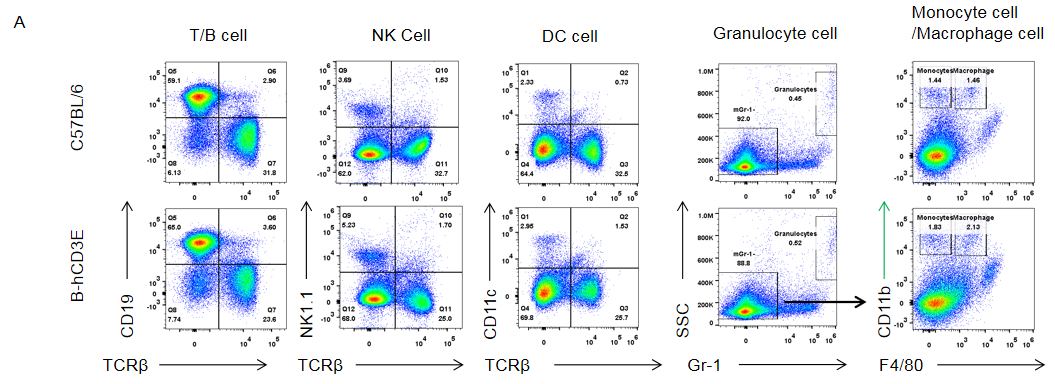
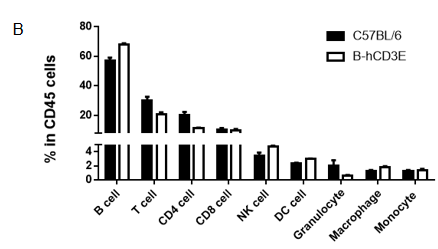
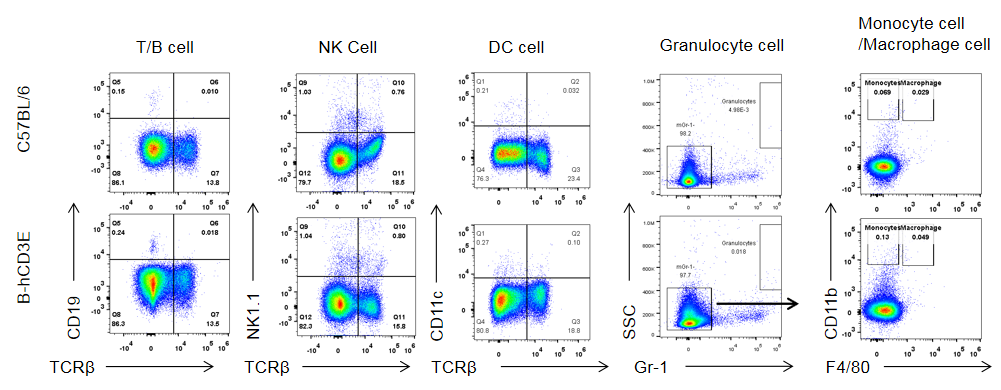
Analysis of spleen leukocytes subpopulation in B-hCD3E mice
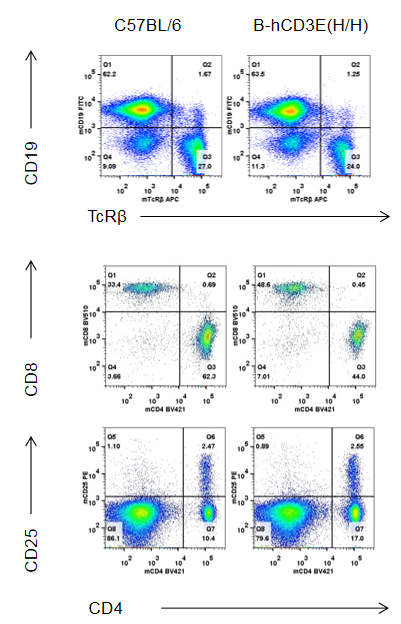
Analysis of thymus leukocytes subpopulation in B-hCD3E mice
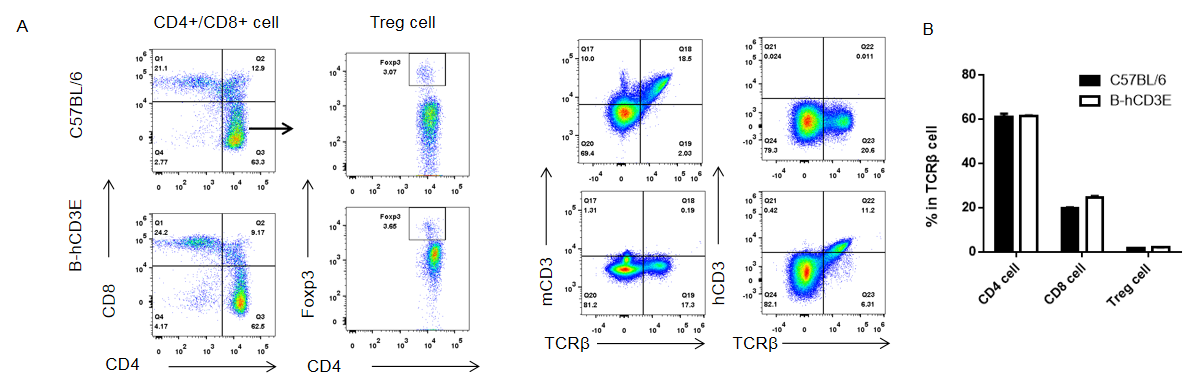
Analysis of thymus T cell subpopulations by FACS. Thymocytes were isolated from female C57BL/6 and B-hCD3E mice (n=6, 6 week-old). Flow cytometry analysis of the thymocytes was performed to assess leukocyte subpopulations. A. Representative FACS plots. Single live CD45+ cells were gated for CD3 T cell population and used for further analysis as indicated here. B. Results of FACS analysis. Percent of CD8, CD4, and Treg cells in homozygous B-hCD3E mice were similar to those in the C57BL/6 mice, demonstrating that introduction of hCD3E in place of its mouse counterpart does not change the overall development, differentiation or distribution of these T cell sub types in thymus. Values are expressed as mean ± SEM. DN: double negative. DP: double positive.
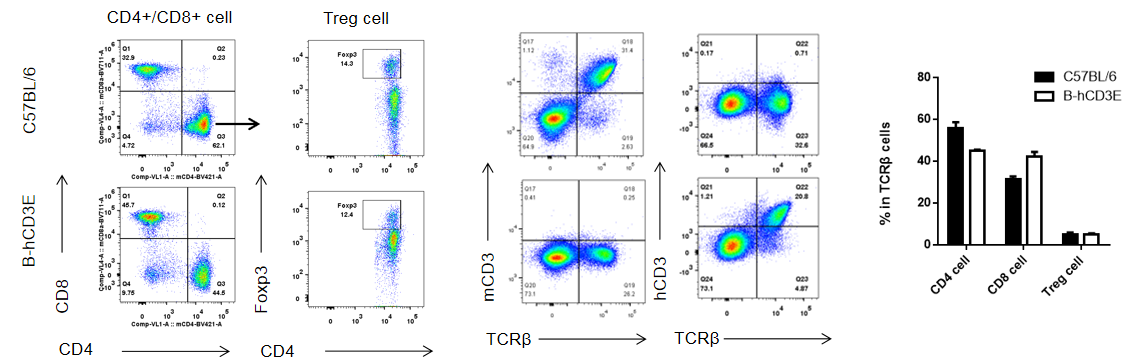
Analysis of B and T cell subpopulation by FACS – Spleen, PBMC, Lymph node
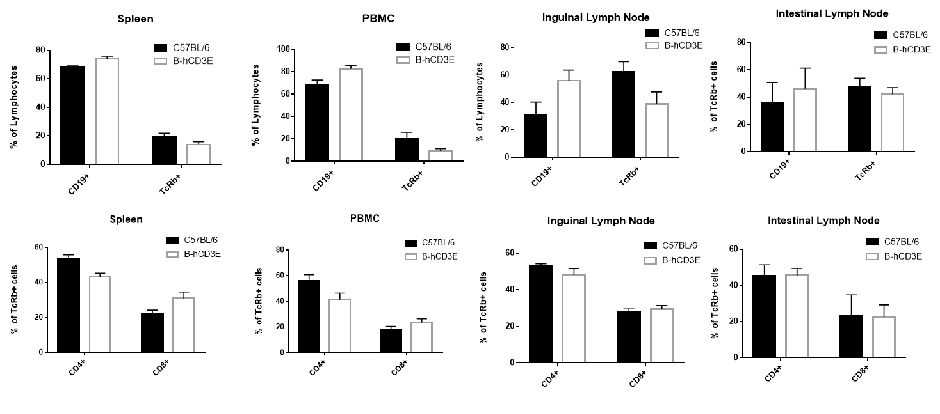
Lymphocytes were isolated from spleen, peripheral blood and lymph node of C57BL/6 and B-hCD3E mice (n=4). Flow cytometry analysis was performed to assess Lymphocytes subpopulations. Single live cells were gated for CD45 population and used for further analysis as shown here. The Percentage of T, B, cells in B-hCD3E mice was similar to that of C57BL/6 mice.
Analysis of T cell activation stimulated with anti-CD3 antibody in vitro
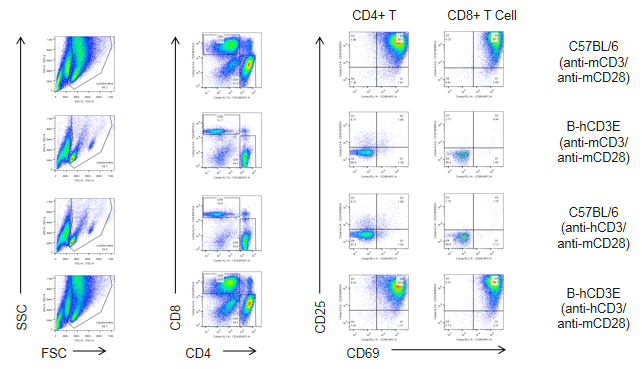
T cells (2.5×106) were isolated from splenocytes of C57BL/6 and B-hCD3E mice (n=4), and were incubated in the presence of anti-CD3E antibody (2ug/ml) and anti-mCD28 antibody (5ug/ml) for 48h. T cell proliferation was tested by flow cytometry. T cell activation in B-hCD3E mice was significantly up-regulated by anti-hCD3E antibody, similar to the activation level shown in C57BL/6 mice treated with anti-mCD3E antibody.
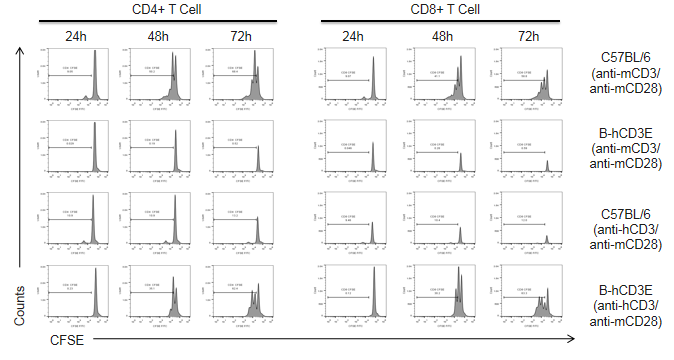
T cells (2.5×106) were isolated from the splenocytes of C57BL/6 and B-hCD3E mice (n=4), and incubated in the presence of anti-CD3E antibody (2ug/ml) and anti-mCD28 (5ug/ml) for 24h, 48h and 72h. T cell proliferation was measured by flow cytometry. T cell activation in B-hCD3E mice was significantly up-regulated by anti-hCD3 antibody, similar to the activation level shown in the anti-mCD3 antibody-treated C57BL/6 mice, indicating that Cd3e humanization in B-hCD3E mice does not affect T cell activation in spleen.
Analysis of T cell activation stimulated with anti-CD3 antibody in vitro
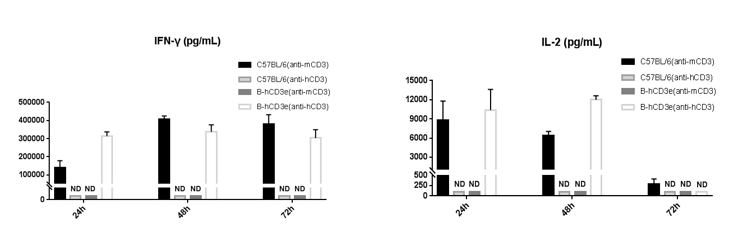
T cells (2.5×106) were isolated from the splenocytes of C57BL/6 and B-hCD3E mice (n=4), and incubated in the presence of anti-CD3E antibody (2ug/ml) and anti-mCD28 antibody (5ug/ml) for 24h, 48h and 72h. IFN-γ and IL-2 production were tested using ELISA method. Concentration of IFN-γ and IL-2 in B-hCD3E mice was similar to that of C57BL/6 mice, indicating that Cd3e humanization in B-hCD3E mice does not affect T cell activation in spleen.
Analysis of T cell activation stimulated with anti-CD3 antibody ex vivo
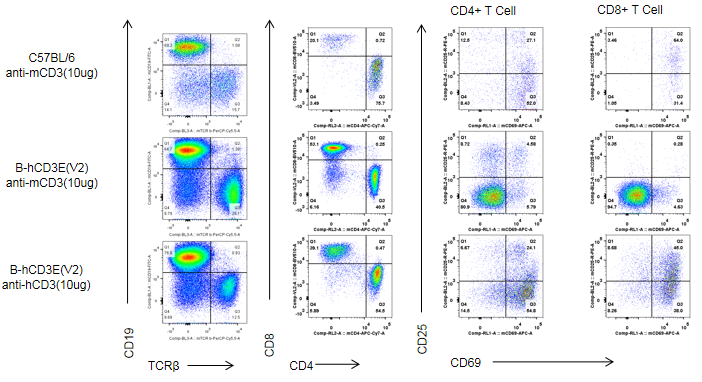
C57BL/6 and B-hCD3E mice were injected intraperitoneally with anti-CD3E antibody (10ug/mouse). After 24h, T cells were isolated from splenocytes of C57BL/6 and B-hCD3E mice (n=4). T cell proliferation was measured by flow cytometry. T cell activation in B-hCD3E mice was significantly up-regulated by anti-hCD3E antibody, similar to the activation level in the anti-mCD3E antibody-treated C57BL/6 mice, demonstrating that introduction of hCD3E in place of its mouse counterpart does not affect T cell activation in spleen.
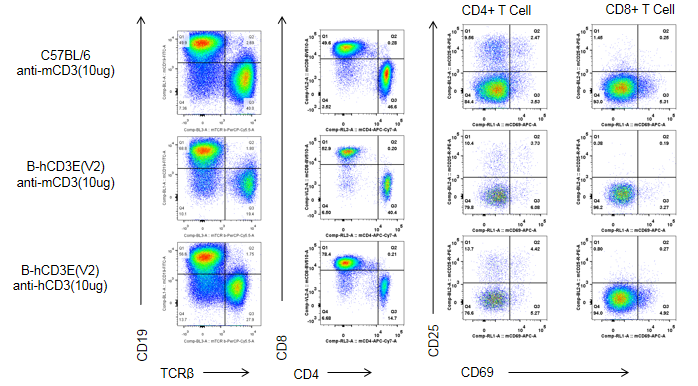
C57BL/6 and B-hCD3E mice were injected intraperitoneally with anti-CD3E antibody (10ug/mouse). After 48h, T cells were isolated from splenocytes of C57BL/6 and B-hCD3E mice (n=4). T cell proliferation was measured by flow cytometry. T cell activation in B-hCD3E mice was significantly down-regulated, similar to the activation level in the anti-mCD3 antibody-treated C57BL/6 mice, demonstrating that introduction of hCD3E in place of its mouse counterpart does not affect T cell activation in spleen.
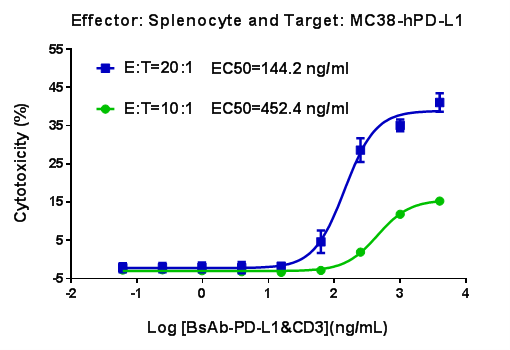
B-hCD3E mouse spleen cells were mixed with MC38-hPD-L1 and various concentrations of CD3-PD-L1 bispecific antibodies were added. The killing activity was detected after 48 hours. When effector cells : target cells (E:T) =10:1,the EC50 of CD3-PD-L1 bispecific antibodies activity was 452.4 ng/mL; When E:T=20:1,the EC50 of CD3-PD-L1 bispecific antibodies activity was 144.2 ng/mL
Serum titers of OVA-specific antibodies
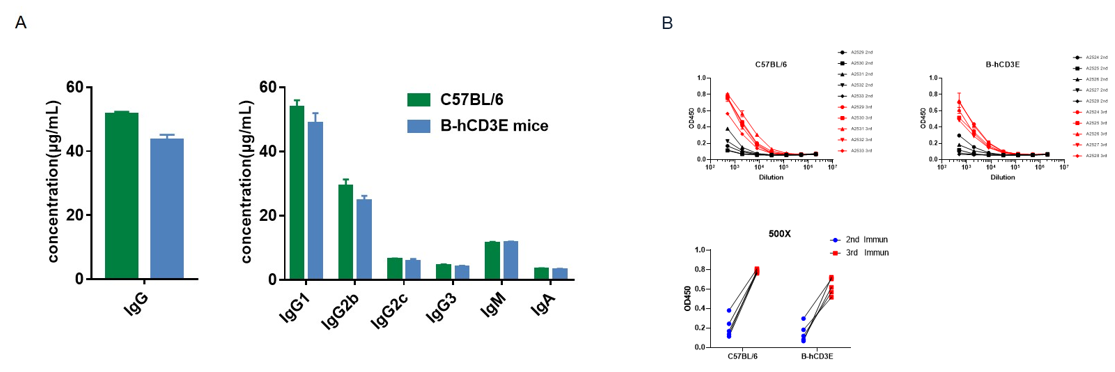
B-hCD3E mice (n=5, 6 week-old) were immunized three times with OVA, 2 weeks apart. Blood samples were collected a week after immunization. (A) Quantification of serum subtypes of mice before immunization. (B) Serum titer test of mice after the second and third immunizations. The levels of OVA-specific antibodies titers of B-hCD3E mice before immunization were similar to those in C57BL/6 mice, and the specific antibody titers in the serum of each mouse were significantly increased after the third immunizations, demonstrating that introduction of hCD3E instead of its mouse counterpart did not affect the humoral immune response of mice. Values are expressed as mean ± SEM.
Blood routine test in B-hCD3E mice
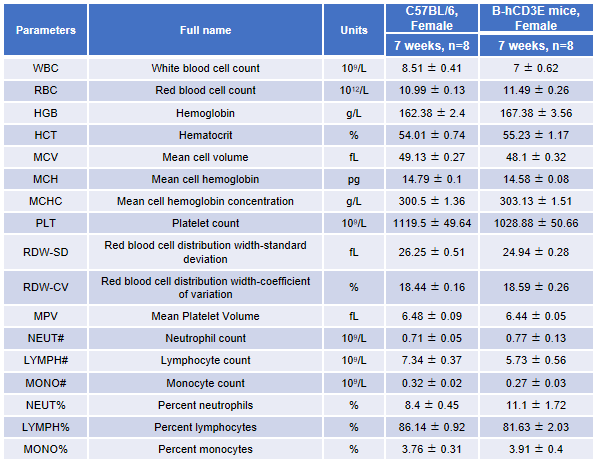
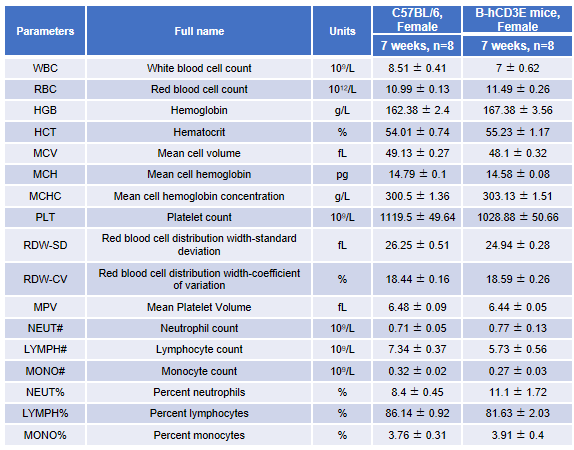
Blood from female C57BL/6 and B-hCD3E mice (n=8, 7-week-old) were collected and analyzed for CBC. There was no differences among any measurement between C57BL/6 and B-hCD3E mice, indicating that introduction of hCD3E in place of its mouse counterpart does not change blood cell composition and morphology. Values are expressed as mean ± SEM.
Serum from female C57BL/6 and B-hCD3E mice (n=8, 7-week-old) were collected and analyzed for levels of ALT (alanine aminotransferase) and AST (aspartate aminotransferase). The measurement results were similar between C57BL/6 and B-hCD3E mice, indicating that introduction of hCD3E in place of its mouse counterpart does not change ALT and AST levels or health of liver. Values are expressed as mean ± SEM.
CD3 Abs efficacy evaluation
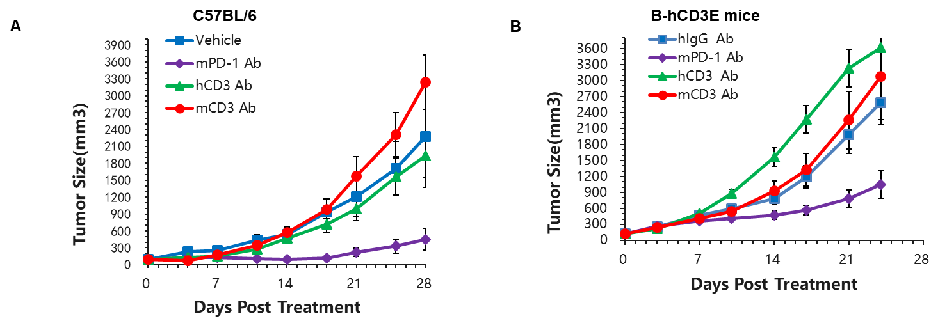
Murine colon cancer MC38 cells were subcutaneously implanted into C57BL/6 (A) and B-hCD3E (B) mice. Mice were grouped when the tumor size was approximately 150±50mm3 (n=5).In B-hCD3E mice, mPD-1 antibody significantly inhibited tumor growth, indicating their T cells function normally. However, in B-hCD3E mice, tumor growth was faster after anti-hCD3E antibody treatment, which may be caused by activation induced cell death (AICD). As a result, the B-hCD3E mouse model is a powerful tool for in vivo CD3 antibody pharmacological efficacy studies.
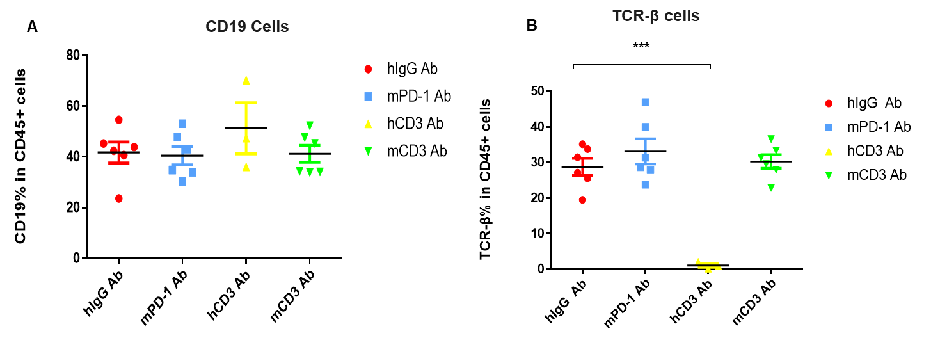
the proportion of T cells was significantly decreased due to the activation induced cell death (AICD) effect caused by CD3E antibody treatment. However, the proportion of T cells has no significant change in the anti-mPD-1 antibody group. (A) Compared with hlgG Ab , there is no significant difference in the percentage of CD19+ cells in total CD45+ cells after hCD3 Ab or mCD3 Ab treatment. (B) Compared with hlgG Ab, the percentage of TCR-β positive cells was significantly decreased after treatment with hCD3 Ab in the humanized mice.
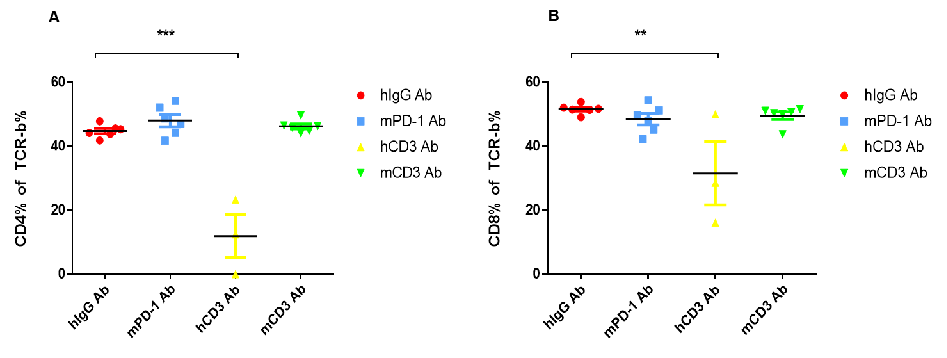
(A) Compared with hlgG Ab, the percentage of CD4+ T cells in the blood decreased significantly after hCD3E Ab treatment. (B) Compared with hlgG Ab, the percentage of CD8+ T cells in the blood was significantly reduced after hCD3E Ab treatment.
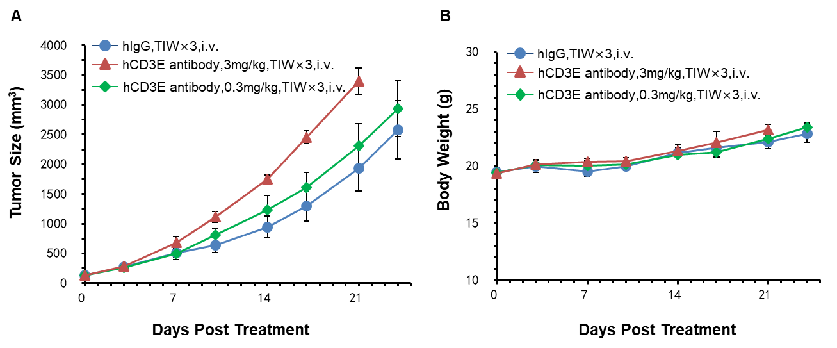
Murine colon cancer MC38 cells were subcutaneously implanted into B-hCD3E mice. Mice were divided into control and treatment groups(n=5) when tumon size was approximately 150±50 mm3. High doses of hCD3E antibodies resulted in faster tumor growth due to activation induced cell death (AICD), confirming that the B-hCD3E mouse model is a powerful tool for in vivo anti-hCD3 antibody pharmacological efficacy study. (A) Tumor average volume ±SEM, (B) Mice average weight±SEM.
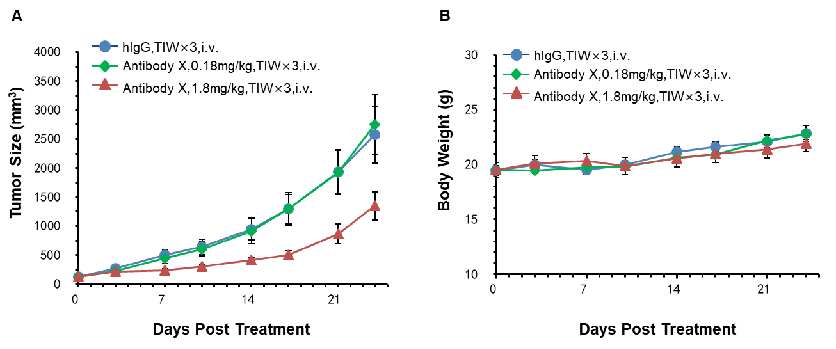
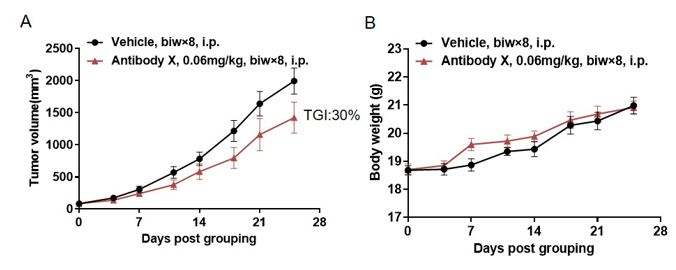
Antitumor activity of Antibody X in B-hCD3E mice. (A) High-dose Antibody X inhibited MC38 tumor growth in B-hCD3E mice(n=5). Murine colon cancer MC38 cells were subcutaneously implanted into homozygous B-hCD3E mice. Mice were grouped when tumor volume reached approximately 100 mm3, at which time they were treated with Antibody X with doses and schedules indicated in panel ; (B) Body weight changes during treatment. As shown in panel A, high-dose Antibody X were efficacious in controlling tumor growth in B-hCD3E mice, demonstrating that the B-hCD3E mice provide a powerful preclinical model for in vivo evaluation of Antibody X . Values are expressed as mean ±SEM
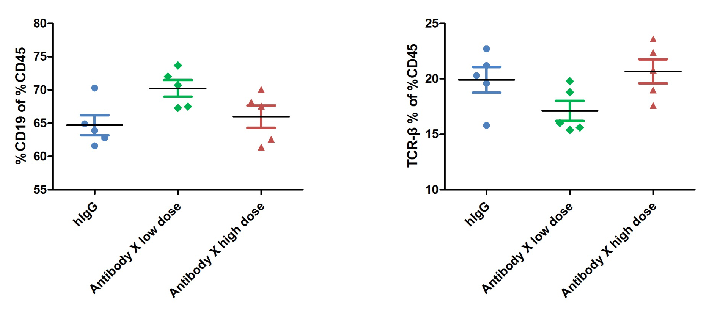
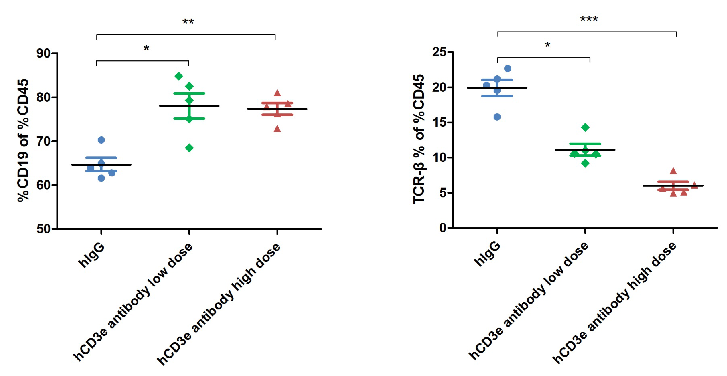
The ratio of B and T cells in the blood of mice was detected by flow cytometry. Lymphocytes were isolated from peripheral blood at the experimental end point. In the treatment group, the ratio of B and T cells did not change significantly.
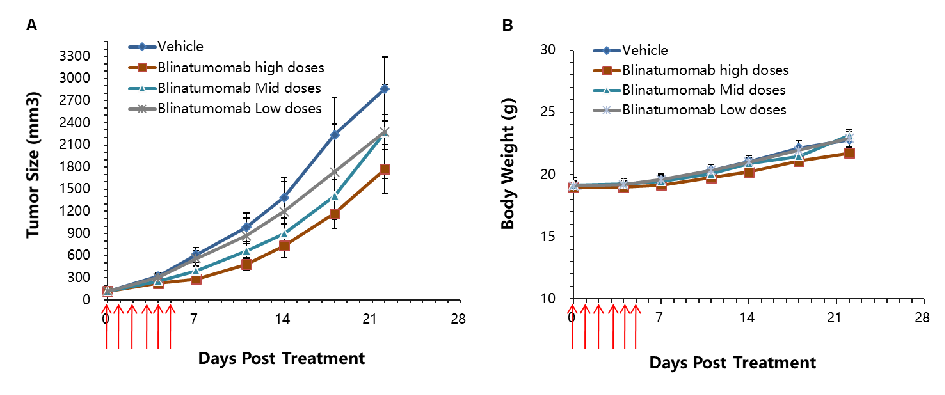
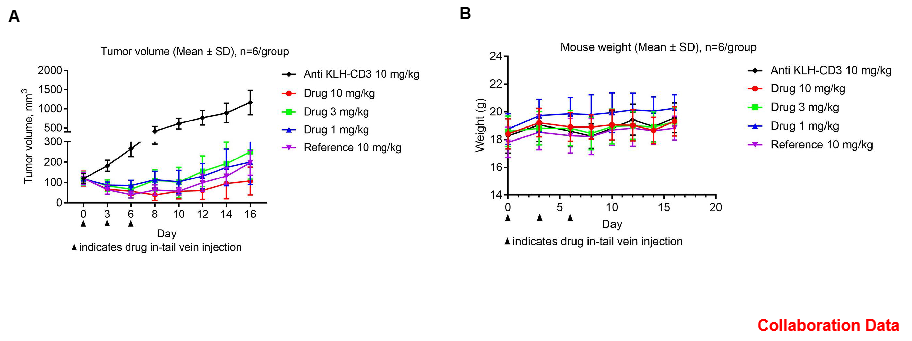
Antitumor activity of CD3E BsAb in B-hCD3E mice. (A) CD3E BsAb inhibited tumor growth in B-hCD3E mice. (B) Body weight changes during treatment. As shown in panel A, CD3E BsAb with different doses were efficacious in controlling tumor growth in B-hCD3E mice, demonstrating that the B-hCD3E mice provide a powerful preclinical model for in vivo evaluation of CD3E BsAb. Values are expressed as mean ± SEM.
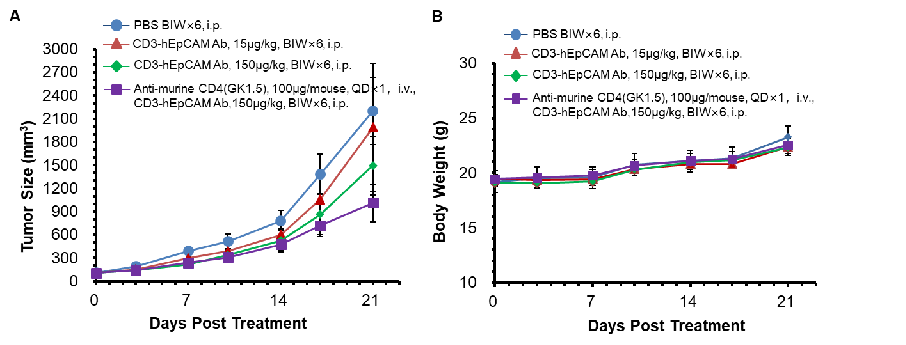
MC38-hEpCAM cells were implanted subcutaneously into B-hCD3E mice. The mice were divided into control and treatment groups when the tumor size was about 100±20 mm3. The results show that the anti-hCD3E/hEpCAM bispecific antibody has a moderate degree of antitumor activity compared to the control group. The data also show that anti-mouse CD4 (a CD4-depleting antibody) enhances the antitumor activity of the bispecific anti-hCD3E/hEpCAM antibody.
In vivo efficacy of CD3 BsAb
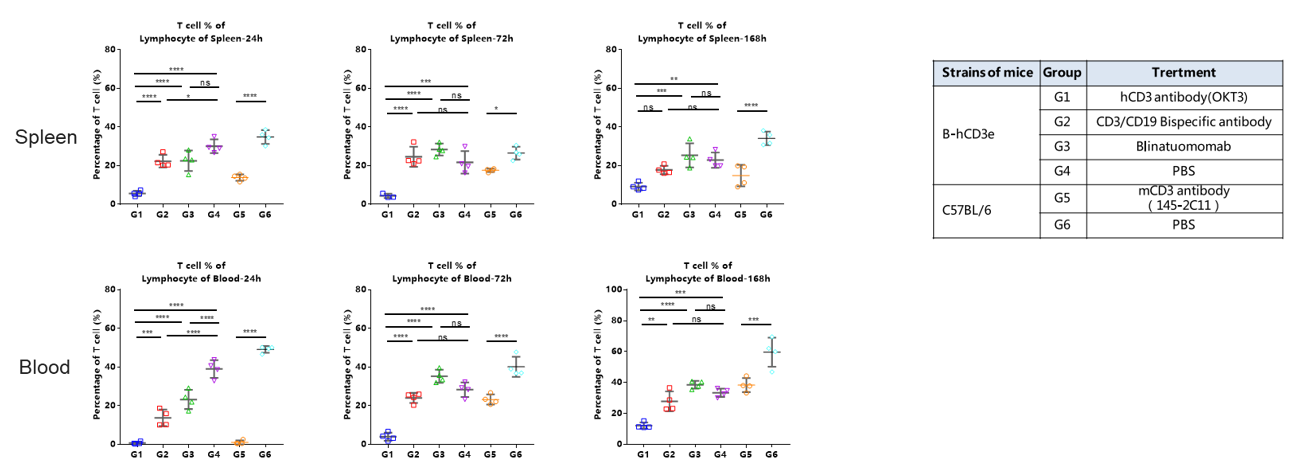
The ratio of T cells in spleen and blood were analyzed at 24h、72 and 168h by flow cytometry.











 京公網安備: 11011502005564號
京公網安備: 11011502005564號